Ozone layer|| ozone layer depletion and it’s mechanism

History
In 1969 Dutch chemist Paul Crutzen published a paper that described the major nitrogen oxide catalytic cycle affecting ozone levels. Crutzen demonstrated that nitrogen oxides can react with free oxygen atoms, thus slowing the creation of ozone (O3), and can also decompose ozone into nitrogen dioxide (NO2) and oxygen gas (O2). Some scientists and environmentalists in the 1970s used Crutzen’s research to assist their argument against the creation of a fleet of American supersonic transports (SSTs). They feared that the potential emission of nitrogen oxides and water vapour from these aircraft would damage the ozone layer. (SSTs were designed to fly at altitudes coincident with the ozone layer, some 15 to 35 km [9 to 22 miles] above Earth’s surface.) In reality, the American SST program was canceled, and only a small number of French-British Concordes and Soviet Tu-144s went into service, so that the effects of SSTs on the ozone layer were found to be negligible for the number of aircraft in operation.Advertisement
Human activities have had a significant effect on the global concentration and distribution of stratospheric ozone since before the 1980s. In addition, scientists have noted that large annual decreases in average ozone concentrations began to occur by at least 1980. Measurements from satellites, aircraft, ground-based sensors, and other instruments indicate that total integrated column levels of ozone (that is, the number of ozone molecules occurring per square metre in sampled columns of air) decreased globally by roughly 5 percent between 1970 and the mid-1990s, with little change afterward. The largest decreases in ozone took place in the high latitudes (toward the poles), and the smallest decreases occurred in the lower latitudes (the tropics). In addition, atmospheric measurements show that the depletion of the ozone layer increased the amount of UV radiation reaching Earth’s surface.
Get exclusive access to content from our 1768 First Edition with your subscription.Subscribe today
This global decrease in stratospheric ozone is well correlated with rising levels of chlorine and bromine in the stratosphere from the manufacture and release of CFCs and other halocarbons. Halocarbons are produced by industry for a variety of uses, such as refrigerants (in refrigerators, air conditioners, and large chillers), propellants for aerosol cans, blowing agents for making plastic foams, firefighting agents, and solvents for dry cleaning and degreasing. Atmospheric measurements have clearly corroborated theoretical studies showing that chlorine and bromine released from halocarbons in the stratosphere react with and destroy ozone.
OZONE LAYERS
Ozone is a form of oxygen. A molecule of ozone contains three oxygen atoms(O3). It is found in th statosphere of the earth.
The ozone layer or ozone shield is a region of Earth‘s stratosphere that absorbs most of the Sun‘s ultraviolet radiation. It contains high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth’s atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9.3 to 21.7 mi) above Earth, although its thickness varies seasonally and geographically.[1]
The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature in the range of 5,500–6,000 K (5,227 to 5,727 °C), except that there was no radiation below a wavelength of about 310 nm at the ultraviolet end of the spectrum. It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually the spectrum of the missing radiation was matched to only one known chemical, ozone.[2] Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The “Dobson unit“, a convenient measure of the amount of ozone overhead, is named in his honor.
The ozone layer absorbs 97 to 99 percent of the Sun’s medium-frequency ultraviolet light (from about 200 nm to 315 nm wavelength), which otherwise would potentially damage exposed life forms near the surface.[3]
In 1976, atmospheric research revealed that the ozone layer was being depleted by chemicals released by industry, mainly chlorofluorocarbons (CFCs). Concerns that increased UV radiation due to ozone depletion threatened life on Earth, including increased skin cancer in humans and other ecological problems,[4] led to bans on the chemicals, and the latest evidence is that ozone depletion has slowed or stopped. The United Nations General Assembly has designated September 16 as the International Day for the Preservation of the Ozone Layer.
Venus also has a thin ozone layer at an altitude of 100 kilometers from the planet’s surface.[5]
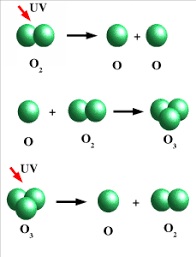
It is formed by the combination of molecular oxygen with atomic oxygen in the presence of UV rays
O2 ———————————————————– O + O
O2 + O ———————————————–O3
Effects of ozone layers
- The ozone layer protects us from severe burns due to uv rays
- It also protects us from skin diseases, cataracts, deficiency of immune power as well as cancer due to the exposure to uv rays.
- It plays a vital role in the balance of weather and temperature on the earth.
- If the ozone comes near the earth’s surface, then it will be very harmful as it causes the burning of throats and the distraction of lungs.
Ozone layer depletion
The thinning of ozone layer or the formation of hole in it then it is known as ozone layer depletion.
Ozone is being depleted by air pollutants. CFC methene oxides of nitrogen , CO2, etc. are the air pollutants that are mainly responsible for the depletion of the ozone layer in the statosphere.
Memory tips
An English scientist J.C. farman found out for the first time of ozone layer depletion over Antartica in 1885 AD.
Mechanism of ozone layer depletion
CFC is the synthetic, Harmful chemical, which is very widely used in refrigerators and air conditioners and coolants: in fire extinguisher, aerosol sprayers as propellants.
Once released in air, these harmful chemicals produce active cholrine (CL and CLO radicals) in the presence of uv rays. These radicals through chain reactions, then destroy by converting it into oxygen as shown in the mechanism given below:
Antartic ozone layer hole
The most severe case of ozone depletion was first documented in 1985 in a paper by British Antarctic Survey (BAS) scientists Joseph C. Farman, Brian G. Gardiner, and Jonathan D. Shanklin. Beginning in the late 1970s, a large and rapid decrease in total ozone, often by more than 60 percent relative to the global average, has been observed in the springtime (September to November) over Antarctica. Farman and his colleagues first documented this phenomenon over their BAS station at Halley Bay, Antarctica. Their analyses attracted the attention of the scientific community, which found that these decreases in the total ozone column were greater than 50 percent compared with historical values observed by both ground-based and satellite techniques.

As a result of the Farman paper, a number of hypotheses arose that attempted to explain the Antarctic “ozone hole.” It was initially proposed that the ozone decrease might be explained by the chlorine catalytic cycle, in which single chlorine atoms and their compounds strip single oxygen atoms from ozone molecules. Since more ozone loss occurred than could be explained by the supply of reactive chlorine available in the polar regions by known processes at that time, other hypotheses arose. A special measurement campaign conducted by the National Aeronautics and Space Administration (NASA) and the National Oceanic and Atmospheric Administration (NOAA) in 1987, as well as later measurements, proved that chlorine and bromine chemistry were indeed responsible for the ozone hole, but for another reason: the hole appeared to be the product of chemical reactions occurring on particles that make up polar stratospheric clouds (PSCs) in the lower stratosphere.
During the winter the air over the Antarctic becomes extremely cold as a result of the lack of sunlight and a reduced mixing of lower stratospheric air over Antarctica with air outside the region. This reduced mixing is caused by the circumpolar vortex, also called the polar winter vortex. Bounded by a stratospheric jet of wind circulating between approximately 50° and 65° S, the air over Antarctica and its adjacent seas is effectively isolated from air outside the region. The extremely cold temperatures inside the vortex lead to the formation of PSCs, which occur at altitudes of roughly 12 to 22 km (about 7 to 14 miles). Chemical reactions that take place on PSC particles convert less-reactive chlorine-containing molecules to more-reactive forms such as molecular chlorine (Cl2) that accumulate during the polar night. (Bromine compounds and nitrogen oxides can also react with these cloud particles.) When day returns to Antarctica in the early spring, sunlight breaks the molecular chlorine into single chlorine atoms that can react with and destroy ozone. Ozone destruction continues until the breakup of the polar vortex, which usually takes place in November.
A polar winter vortex also forms in the Northern Hemisphere. However, in general, it is neither as strong nor as cold as the one that forms in the Antarctic. Although polar stratospheric clouds can form in the Arctic, they rarely last long enough for extensive decreases in ozone. Arctic ozone decreases of as much as 40 percent have been measured. This thinning typically occurs during years when lower-stratospheric temperatures in the Arctic vortex have been sufficiently low to lead to ozone-destruction processes similar to those found in the Antarctic ozone hole. As with Antarctica, large increases in concentrations in reactive chlorine have been measured in Arctic regions where high levels of ozone destruction occur.
Ozone Layer Recovery
The recognition of the dangers presented by chlorine and bromine to the ozone layer spawned an international effort to restrict the production and the use of CFCs and other halocarbons. The 1987 Montreal Protocol on Substances That Deplete the Ozone Layer began the phaseout of CFCs in 1993 and sought to achieve a 50 percent reduction in global consumption from 1986 levels by 1998. A series of amendments to the Montreal Protocol in the following years was designed to strengthen the controls on CFCs and other halocarbons. By 2005 the consumption of ozone-depleting chemicals controlled by the agreement had fallen by 90–95 percent in the countries that were parties to the protocol.
During the early 2000s, scientists expected that stratospheric ozone levels would continue to rise slowly over subsequent decades. Indeed, some scientists contended that, as levels of reactive chlorine and bromine declined in the stratosphere, the worst of ozone depletion would pass. Factoring in variations in air temperatures (which contribute to the size of ozone holes), scientists expected that continued reductions in chlorine loading would result in smaller ozone holes above Antarctica (which since 1992 have spanned more than 20.7 million square km [8 million square miles]) after 2040. The expected increases in ozone would be gradual primarily because of the long residence times of CFCs and other halocarbons in the atmosphere. Total ozone levels, as well as the distribution of ozone in the troposphere and stratosphere, would also depend on other changes in atmospheric composition—for example, changes in levels of carbon dioxide (which affects temperatures in both the troposphere and the stratosphere), methane (which affects the levels of reactive hydrogen oxides in the troposphere and stratosphere that can react with ozone), and nitrous oxide (which affects levels of nitrogen oxides in the stratosphere that can react with ozone).
Merits of ozone layer
The ozone layer of the atmosphere serves to protect life on our planet from the more harmful effects of ultraviolet radiation which is most damaging at the shorter wavelengths of 290-320 nm in the UV-B part of the electromagnetic spectrum. UV-B light readily damages DNA at the molecular level, which ain’t at all good.